Task Force 3 – LiFE, Resilience, and Values for Wellbeing
Abstract
Global health could be much improved if medical technologies were more effectively deployed. The obstacles to better global health are multifaceted, including non-availability of pharmaceuticals in low-income and remote regions, high prices impeding access, severe shortages of diagnostic capabilities, and lack of coordinated efforts towards fighting diseases at the population level. Being the main sources of worldwide development support and home to the most relevant research and development (R&D) and manufacturing, the G20 countries are well placed to address these problems holistically. One way forward is to create more effective incentives that tie rewards for developing, manufacturing, and delivering pharmaceuticals to the health gains achieved with them. This approach could be launched and tested through an Ubuntu Health Impact Fund (UHIF) pilot focused on Africa, where health deficits are most severe. “Ubuntu” is an African word meaning “humanity to others” and signifies how we all live in a global community in which interdependence is not only inevitable but beautiful.
1. The Challenge
Humanity has made enormous progress over the last 60 years, as exemplified by a tripling of global income per person in real terms, a massive reduction in child mortality, from over 21 percent to under 4 percent today, and an astonishing increase in average life expectancy from 47 to 71 years.[1] This unprecedented achievement is, however, unevenly shared: national average annual incomes range from US$660 to US$117,000, national infant mortality rates range from 12.7 to 0.21 percent, and national life expectancies range from 52 to 84 years.[2] These shocking inequalities have motivated the adoption of development goals such as the recent Agenda 2030, with its inspiring motto, Leave No One Behind.[3]
Pharmaceuticals are among humanity’s greatest achievements. They have facilitated dramatic improvements in health and longevity as well as huge cost savings through reduced sick days and hospitalisations.[4] Yet, the pharmaceutical sector is a key driver of persistent inequality, enabling affluent people to lead healthy lives into their nineties, even as billions of poor people lack the treatments they need. Four main drivers of this inequality are as follows:
- diseases affecting mainly the poor attract substantially less R&D investment, leading to a lack of available treatments;[5]
- existing pharmaceuticals are less likely to be stocked and offered for sale in impoverished areas;[6]
- the poor have less money to buy the medicines they need;[7] and
- pharmaceuticals are often priced higher in poor countries that, lacking manufacturing plants, must import them at high costs.[8]
We support the G20’s commitment to mitigate these severe inequalities.
Unequal pharmaceutical provision is an effect of economic inequality. Affluent people can, often through good health insurance, afford quality diagnostics and medical treatments. Therefore, pharmaceutical firms have ample incentives to develop and supply effective products for the needs of affluent populations. Because such firms cannot earn much money from poor patients, they spend little on developing and supplying effective products to address the needs of the poor. As a result, the poor suffer a much higher burden of disease which, in turn, tends to aggravate their poverty and deepen global inequality.
One might think that, even if they are focused on serving the affluent, pharmaceutical firms would have strong reasons to address the needs of the poor, at least in regard to infectious diseases. As recent experience shows, an effective response to a pandemic requires a global strategy of disease containment and suppression, which cannot succeed if it excludes poor populations and thus allows them to proliferate the disease and even become breeding grounds for the emergence of new, possibly drug-resistant, disease variants.
Compelling as this thought is, it is not reflected in the current incentives of pharmaceutical firms. These firms earn their income by serving affluent people at the individual level, through diagnosing and treating their diseases or by individually vaccinating them against disease. They earn nothing from protecting affluent people at the population level, to ensure that they are not endangered by the disease in the first place. Were a pharmaceutical firm to implement a successful strategy for suppressing and eradicating its target disease, keeping large segments of humanity out of harm’s way, it would reduce its future opportunities for profits from treating this disease and thus be penalised for its success.
We are thus faced with the challenge of conceiving better incentives for the pharmaceutical sector so that firms can sustainably protect the health of the poor as well and find it worthwhile to fight infectious diseases at the population level with an eye to the containment, suppression, and ultimate eradication of such diseases. The goal is better alignment of pharmaceutical company earnings with the impact of their efforts on human health.
Reflecting on this challenge, our attention is drawn to Africa, which displays the most dramatic underperformance of the pharmaceutical sector relative to its potential. Africa is the continent with the highest incidence of severe poverty. It also has the largest disease burden from infectious diseases, which pharmaceutical firms tend to neglect and not combat strategically at the population level. As a result, despite having 17 percent of the global population, Africa accounts for 25 percent of the global disease burden, 91 percent of HIV-positive children, and 95 percent of global malaria cases and 96 percent of global malaria deaths.[9]
With an import ratio of over 80 percent, Africa is heavily dependent on the import of pharmaceutical supplies.[10] This dependence is most severe in Sub-Saharan Africa and became pronounced during the pandemic, when Africans were among the last to receive urgently needed COVID-19 vaccines and consequently suffered severe supply chain disruptions that led to widespread shortages of many essential medicines, as documented in Kenya and Rwanda, among others.[11]
Lack of local production leads to high prices. Long pharmaceutical supply chains with multiple intermediaries ensure that the prices at which medicines are sold in Africa are among the highest in the world.[12] Given that African populations are among the poorest, such high prices greatly impede access to vital medicines which, in turn, entails substantially greater burdens of disease and premature mortality.
High prices are also the key driver of the counterfeit medicines business, which is worth an estimated US$200 billion annually, with an African share of 42 percent.[13] Counterfeit or ‘fake’ drugs have been proven to compromise the treatment of chronic and infectious diseases, causing disease progression, drug resistance, and even death.[14]
Africa’s high disease burden slows its development in all dimensions, with adverse effects on initiatives such as Compact with Africa. This headwind is aggravated by the fact that, lacking local production, Africa misses out on participating in the medicine market, which is a rapidly growing and important economic sector that accounts for well over 2 percent of the gross world product.[15] Strengthening diagnostic capabilities as well as pharmaceutical manufacturing and competent delivery of products would be a major boon to the continent’s overall development.
Recognition of these problems has spawned multiple efforts to advance pharmaceutical manufacturing in Africa. The African Union’s heads of state proposed a plan in 2005, which led to the Pharmaceutical Manufacturing Plan for Africa.[16] A further, more detailed strategy of over hundred pages was formulated in 2012 by the African Union Commission and the United Nations Industrial Development Organization.[17] This document identified numerous obstacles to production in Africa, including:
- Access to finance
- Cost of implementation of Good Manufacturing Practices
- Small local markets
- Shortage of appropriately trained staff
- Weak pharmaceutical regulation, and
- Underdeveloped supporting industries
An important step to addressing many of these problems was taken with the establishment of the African Medicines Agency (AMA) in 2021, which could promulgate common standards and regulations, coordinate reviews of clinical trial applications, evaluate medical products and pharmaceutical manufacturing plants, and provide a clearinghouse for information about products authorised for sale.[18] The operationalisation of the AMA is still in progress.[19]
In 2022, the African Development Bank approved the creation of the African Pharmaceutical Technology Foundation to provide financing and technical support for manufacturing in this vital sector, with a focus on diseases most prevalent in Africa. In justifying the allocation of resources to this program, the bank’s president, Dr. Akinwumi Adesina, explained that “Africa can no longer outsource the healthcare security of its 1.3 billion citizens to the benevolence of others.”[20]
In effect, there has been a consistent effort to advance pharmaceutical manufacturing in Africa, and many components are coming together. However, there is also a need to provide financial support to help build up such manufacturing capacity, so that efforts to advance the AMA and the African Pharmaceutical Technology Foundation will be more productive.
2. The Role of the G20
Massive avoidable global health deficits fall most clearly under the remit of the Sherpa Track’s Health Working Group but also engage the aspirations of its Development Working Group. Our proposal to pioneer a new way of incentivising and funding the development and effective deployment of socially beneficial health innovations should also be of interest to the Finance Track’s Joint Finance and Health Task Force.
Focusing on Africa as the region to try out this new approach harmonises with long-standing G20 support for industrialisation in the continent as well as for African initiatives such as the Pharmaceutical Manufacturing Plan for Africa, the African Pharmaceutical Technology Foundation, and the AMA. The declaration of the G20 Leaders’ Summit in Hangzhou, China, in September 2016, launched the G20 Initiative on Supporting Industrialization in Africa and Least Developed Countries.[21] In 2017, Compact with Africa was initiated with support from the G20.[22] The latest report of the G20 Africa Advisory Group concludes:
While economic reforms carried out by African Compact members remain key to attracting investment and boosting prosperity, we explicitly call upon our G20 partners to strengthen their engagement. This should include investment promotion for Compact countries, namely by enhancing public-private dialogue, and also the promotion of joint funding, including adequately resourced multi-donor trust funds which will facilitate reform implementation in Compact countries.[23]
There are substantial total bilateral aid flows to support industrial development. The chart below shows total aid to industry in 2015 US$ across all countries (not just Africa).
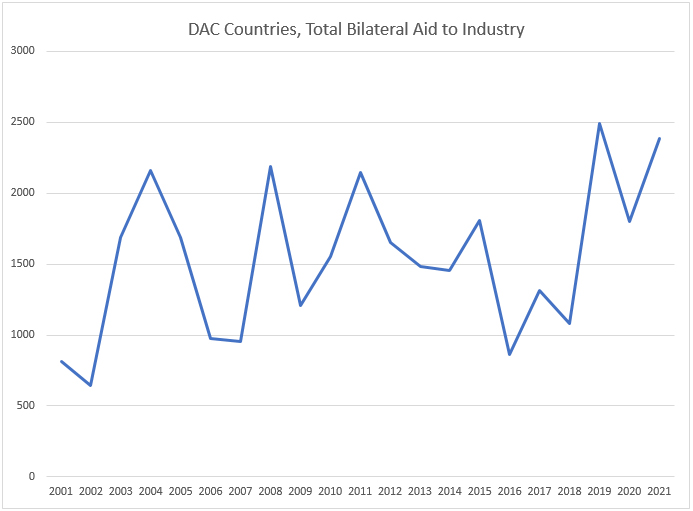
Source: OECD. Stat; data in millions of 2015 US$.
It is in the spirit of these G20 efforts and commitments that we are proposing the Ubuntu Health Impact Fund. African countries have pointed to the importance of the pharmaceutical industry and have been building the institutional structures needed to support the industry. The COVID-19 pandemic reflects the urgency for a fairer, better-balanced distribution of pharmaceutical production throughout the world. These are strong reasons for targeting some of the industrialisation support envisioned by the G20 for the development of Africa’s pharmaceutical industry.
How can this be done in a way that is responsive to the need to better align industry incentives with the imperative to improve population health?
3. Recommendations to the G20
Massive reductions in global disease burden are possible by better aligning the rewards for developing and delivering pharmaceuticals with their impact on health. It is for this purpose that we have proposed the establishment of a Health Impact Fund that would give pharmaceutical innovators the option to exchange some of their monopoly privileges for impact rewards proportionate to the health gains achieved through their innovations.[24] The G20 could help test and refine this approach by supporting a pilot in Africa, which would demonstrate the feasibility of health impact assessment, the willingness of pharmaceutical firms to be paid for performance, and the cost-effectiveness of the impact fund approach.
The proposed Ubuntu Health Impact Fund (UHIF) would reward pre-selected pharmaceutical firms that are willing to inaugurate the manufacture of a specific pharmaceutical in Africa and sell it in a self-chosen African region at or below the globally lowest commercial price for this product. The UHIF would do so by dividing a fixed pool of reward money among the participating firms according to the health gains generated through their respective products in their target areas over a three-year period. Here, health benefits would include externalities such as third-party health benefits to persons whose risk of infection is reduced.
The UHIF’s innovative financing mechanism would promote a vibrant and competitive pharmaceutical manufacturing sector that meets the needs of Africa’s population by ensuring adequate supply that is competently delivered at prices that patients and other payers can afford. It would promote pharmaceutical manufacturing, access to important medicines in Africa, and strategic deployment of such medicines, especially at the primary healthcare level among neglected populations. It would also encourage economic and infrastructural investment in Africa that would not be solely focused on the health sector but on the development of Africa as a whole.
Such an impact-focused reward system would incentivise participating pharmaceutical firms not merely to sell their product but to make it effective through proper diagnostics and instructions, without which pharmaceuticals can be, and often are, useless or even harmful to human health. Such firms would also want to invest in diagnostics for the sake of obtaining the data required for effectively fighting the target disease at the population level. Rapid diagnostic tests can be crucial for identifying emerging health threats, such as infectious disease outbreaks, and for being able to distinguish among diseases with similar symptoms but different treatment regimens. Rapid and accurate responses to disease, in turn, magnify a pharmaceutical’s health benefits and, hence, the earnings of the providing firm. The UHIF would thus incentivise the production of important pharmaceuticals in Africa for sale at affordable prices to patients who need them with the central purpose of cost-effective reduction of Africa’s disease burden.
To be eligible for participation in the UHIF pilot, a pharmaceutical firm or consortium of such firms would have to:
- manufacture in Africa a pharmaceutical product that has never been manufactured in the continent,
- sell the manufactured supply of this product in a specified region of Africa (or all of Africa) at a qualifying price, and
- collaborate with the UHIF in the transparent and reliable assessment of the health gains achieved by supplying the product in question within the target region.
An expert committee would select the best four to five proposals based on their anticipated cost-effectiveness (health gains relative to investment), innovative potential, suitability for reliable and inexpensive health impact assessment, anticipated benefits for poorer population segments, and degree of African ownership and control. While final reward allocations would be calculated at the end of the three-year implementation period, the committee would have the option to support resource-poor proponents with advance payments that would later be subtracted from their final award.
By supporting the UHIF, the G20 would leverage its convening power to highlight the importance of pharmaceutical manufacturing in Africa and other low- and lower-middle income countries, continuous with G20 initiatives on industrialisation and other related initiatives. Putting a spotlight on global issues is a comparative advantage of the G20. This can have snowball effects on countries and organisations, which can then be spurred to action.
The idea of Ubuntu is also expressed in the theme of India’s G20 presidency, “Vasudhaiva Kutumbakam”—“One Earth, One Family, One Future”.[25] In this spirit, the Ubuntu Health Impact Fund pilot is designed to rely on collaboration while supporting access to medicines even for the poorest among us. Its implementation would achieve significant health gains for Africans; enhance African capacities in pharmaceutical innovation, manufacturing, and delivery; and generate crucial learning towards health system strengthening in Africa and the eventual establishment of a global Health Impact Fund.
Endnotes
[1] “GDP Per Capita (Constant 2015 US$),” World Bank Open Data, accessed May 1, 2023.; Max Roser, Hannah Ritchie, and Bernadeta Dadonaite, “Child and Infant Mortality,” Our World in Data, May 10, 2013; Max Roser, Esteban Ortiz-Ospina, and Hannah Ritchie, “Life Expectancy,” Our World in Data, May 23, 2013.
[2] Lucas Chancel and Thomas Piketty, “Global Income Inequality, 1820-2020: The Persistence and Mutation of Extreme Inequality,” Journal of the European Economic Association 19, no. 6 (October 2021): 3025–3062.
[3] “Transforming Our World: The 2030 Agenda for Sustainable Development,” United Nations, 2015.
[4] Peter Hogg, “Top 10 Most Important Drugs in History,” Proclinical, January 18, 2022.
[5] Eliana Barrenho, Marisa Miraldo, and Peter C. Smith, “Does Global Drug Innovation Correspond to Burden of Disease? The Neglected Diseases in Developed and Developing Countries,” Health Economics 28, no. 1 (12 November 2018): 123–143.; Javad Moradpour and Aidan Hollis, “Patient Income and Health Innovation,” Health Economics Letter 29, no. 12 (September 2020): 1795–1803.
[6] Tefo Phaege, “Dying from Lack of Medicines,” Africa Renewal, United Nations, 2016.
[7] Swathi Iyengar, Kiu Tay-Teo, Sabine Vogler, Peter Beyer, Stefan Wiktor, Kees de Joncheere, and Suzanne Hill, “Prices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis,” PLOS Medicine, May 31, 2016.; Melissa Barber, Dzintars Gotham, Giten Khwairakpam and Andrew Hill, “Price of a Hepatitis C Cure: Cost of Production and Current Prices for Direct-Acting Antivirals in 50 Countries,” Journal of Virus Eradication 6, no. 3 (September 2020): 100001.
[8] Michael Conway et al., “Should Sub-Saharan Africa Make Its Own Drugs?,” McKinsey, January 10, 2019.
[9] Theo Vos et al., “Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019,” The Lancet 396, no. 10258 (October 2020): 1204–1222.
[10] Janet Byaruhanga, “How Africa Can Manufacture to Meet Its Own Pharmaceutical Needs | Africa Renewal,” Africa Renewal, United Nations, September 4, 2020.
[11] Alison Buckholtz, “Inside Africa’s Push to Make Its Own Medicines,” International Finance Corporation, World Bank, June 2021.; Edwine Barasa et al., “Assessing the Indirect Health Effects of the COVID-19 Pandemic in Kenya,” Center For Global Development, March 2021.; Theogene Uwizeyimana et al., “Drug Supply Situation in Rwanda during COVID-19: Issues, Efforts and Challenges,” Journal of Pharmaceutical Policy and Practice 14, no. 1 (January 2021).
[12] Michel Sidibé, Abdoul Dieng, and Kent Buse, “Advance the African Medicines Agency to Benefit Health and Economic Development,” British Medical Journal (BMJ) (February 2023): 386.
[13] Kalliroi S. Ziavrou, Stephen Noguera, and Vassiliki A. Boumba, “Trends in Counterfeit Drugs and Pharmaceuticals Before and During COVID-19 Pandemic,” Forensic Science International 338 (July 2022): 111382.
[14] Gillian J. Buckley, and Lawrence O. Gostin, “Countering the Problem of Falsified and Substandard Drugs,” National Academies Press, May 20, 2013.
[15] “Global Medicine Spending and Usage Trends: Outlook to 2025,” IQVIA, April 28, 2021.
[16] “Decision on the Interim Report on HIV/AIDS, Tuberculosis, Malaria and Polio,” Assembly of the African Union Fourth Ordinary Session 30–31 January, 2005, Abuja, Nigeria.
[17] “UNIDO | United Nations Industrial Development Organization,” African Union & UNIDO, 2012.
[18] “African Medicines Agency (AMA),” AUDA-NEPAD, accessed 1 May 2023.
[19] Sara Jerving, “Next up for the African Medicines Agency: Appoint a Director General,” Devex, January 26, 2023.
[20] “African Development Bank’s Board Approves Landmark Institution: Establishment of African Pharmaceutical Technology Foundation to Transform Africa’s Pharmaceutical Industry,” African Development Bank Group, June 27, 2022.
[21] “G20 Initiative on Supporting Industrialization in Africa and Least Developed Countries,” G20 Research Group, October 2016.
[22] “About the Compact with Africa,” G20 Compact with Africa, 2022.
[23] “G20 Compact with Africa: Chairs’ Conclusions Africa Advisory Group Video Conference,” G20 Compact with Africa, April 15, 2021.
[24] “Health Impact Fund,” Incentives for Global Health, accessed 1 May 2023.; Thomas Pogge, “Just Rules for Innovative Pharmaceuticals,” Philosophies 7, no. 4 (July 2022): 79.
[25] “Logo and Theme,” G20 India 2023, accessed 1 May 2023.





